The Nurse Reviews the Clients Arterial Blood Gas and Find the Ph Is 731 Pco2 Is 50 and Hco3 Is 23
This content was published in 2011. We exercise not recommend that you lot make any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Maintaining the pH of claret is essential for normal bodily role. Even so, numerous clinical scenarios can consequence in disruption of the trunk's acrid-base of operations rest. Monitoring of acid-base of operations residue is done by testing patients' arterial blood gases (ABGs). The results of ABG testing will often influence the treatment that patients receive. Therefore, a basic understanding of how to interpret ABG results can be useful for pharmacists to help them analyze the clinical picture.
The basics of acid-base rest
The optimal physiological pH of extracellular fluid is 7.35–7.45. A pH outside this range can cause protein denaturation and enzyme inactivation [1]. Considering pH is a logarithmic scale, a pocket-sized change in pH reflects a large change in hydrogen ion (H+) concentration [1]. The following equilibrium equation is crucial to understanding acid-base balance:
H2O + CO2↔H2CO3↔HCO3 –+H+
This equation shows that carbon dioxide (CO 2 ) in claret dissolves to grade carbonic acrid (HiiCO3), which dissociates to class acidic H+ (which can then combine with physiological bicarbonate to push the equation dorsum to the left). Claret pH depends on the balance of CO2 and HCOthree — a change in the amount of COii will not lead to a change in pH if information technology is accompanied by a change in the amount of HCO3 – that preserves the balance (and vice versa) [2]. It is the renal and respiratory systems that are responsible for maintaining the pH of the blood.
Respiratory mechanisms
One way that the torso controls the pH of extracellular fluid is by increasing or decreasing the rate and depth of respiration and thereby the amount of COii expelled (ie, slow, shallow breathing retains more CO2 than fast, deep breathing).
Renal (metabolic) mechanisms
Another manner that the body tin control pH is via the kidneys, which occurs by either:
- Excretion of H+
- Renal tubular reabsorption of HCO3–
The kidneys can adjust the amount of H+ and HCO3– that is excreted in the
urine in response to metabolic acid product.
Bounty
When acidosis or alkalosis occurs (either through respiratory or renal mechanisms), the reverse system will attempt to rectify this imbalance; this is termed "compensation". For example, if the kidneys fail to excrete metabolic acids, ventilation is adjusted in order to eliminate more COii [two].
Information technology is important to note that compensatory changes in respiration can
occur over minutes to hours, whereas metabolic responses take hours or days to develop[three].
Buffers
The body has three main buffers that minimise any changes in pH that occur when acids or bases are added, namely haemoglobin, HCO3– and proteins. Haemoglobin is six times more powerful every bit a buffer than proteins[ane]. Even so, HCO3– is the most important buffer in the blood and is the dominant buffer in the interstitial fluid. The intracellular fluid uses proteins and phosphate to buffer pH[iii]. At an intracellular
level buffering occurs instantly, but the effect is small-scale.
Arterial blood gas sampling
Monitoring ABGs can be useful to:
- Appraise the effectiveness of pulmonary gas exchange;
- Place the presence of metabolic acidosis and alkalosis;
- Identify critically unwell patients requiring urgent intervention;
- Guide treatment and monitor response.
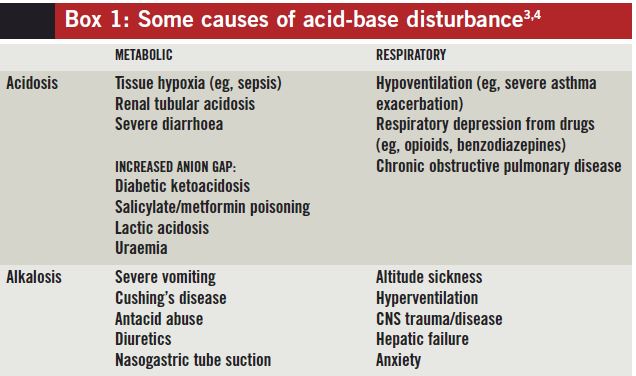
Some causes of acid-base disturbances can be establish in Box 1.
Box i: Some causes of acid-base disturbance[3,4]
The following are the unremarkably reported parameters of ABG results (meet
Box two for the normal reference ranges):
- pH — to determine whether a patient's blood pH is within physiological range;
- PaCO2 and PaOtwo — the partial pressures of CO2 and oxygen in arterial blood, respectively HCO3 – — indicates how much HCO3 – is in the blood (and is therefore available every bit a buffer);
- Base excess (or deficit) — a measure of the backlog or deficiency of base in the claret; past definition, information technology is the amount of base (in mmol) that would correct one litre of blood to a normal pH (if an backlog, this is the corporeality of base needed to be removed for a normal pH, or if a deficit, the amount required to be added);
- Lactate — the end product of anaerobic glycosis (a rise indicates poor oxygenation and perfusion of tissues).
Other parameters ordinarily found on ABG reports are: haemoglobin, glucose and electrolytes (sodium, potassium, chloride and ionised calcium).
Interpreting the results
ABGs tin exist interpreted using a stepped approach:
Step 1 — check the pH
The pH should be assessed first. A pH of less than seven.35 indicates acidosis and a pH greater than 7.45 indicates alkalosis.
Step 2 — check the HCO3 – and PaCO2
Having determined if the patient is acidotic or alkalotic, cheque the HCO3– and the PaCO2 to classify the results equally follows:
- Metabolic acidosis: patients who are acidotic and have a HCO3– <22 (base excess <–2);
- Respiratory acidosis: patients who are acidotic with a PaCO2 >6;
- Metabolic alkalosis: patients who are alkalotic with a HCO3 – >28 (base of operations excess >+2);
- Respiratory alkalosis: patients who are alkalotic with a PaCO2 <iv.7.
Information technology is possible for patients to take a mixed respiratory and metabolic alkalosis or acidosis. This occurs when principal respiratory and main metabolic disturbances exist simultaneously. If the two processes oppose each other, pH derangement will be minimised (see step 3). However, ii processes that crusade pH to move in the same direction may atomic number 82 to profound acidosis or alkalosis[2].
Box 2: Normal results[ii]

Pace 3 — Bank check for bounty
Check to see if the patient is compensating for his or her acid-base imbalance. Patients may partially or fully compensate for an acrid-base imbalance past the "contrary" machinery; for case metabolic acidosis
volition be compensated for with respiratory alkalosis. This may create some plain normal results amidst some deranged ones.
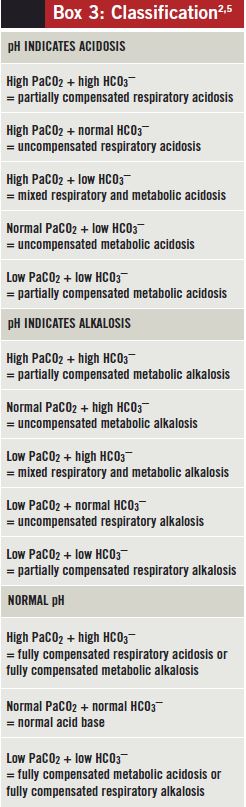
When interpreting acid-base status, it is important e'er to take the clinical context into account. For instance, if presented with ABG results showing a normal pH, low PaCO2 and low HCO3 – in a diabetic patient with high levels of ketones in urine the near likely primary disorder is metabolic acidosis (diabetic ketoacidosis), rather than respiratory alkalosis (see Box 3).
Footstep 4 — Calculate the anion gap
For a patient with metabolic acidosis it can be useful to calculate the anion gap because this can give some indication of the underlying cause of the acrid-base of operations imbalance.
The anion gap is the divergence between the measured positively charged cations (sodium [Na+] and potassium [K+]) and the negatively charged anions (chloride [Cl–] and HCOthree –)[one]. The post-obit equation can be used to estimate the anion gap:
([Na + ] + [Thou+]) – ([Cl–] + [HCO3 –])
An increased anion gap indicates backlog acid from the anions that are unmeasured (eg, ketones or lactate)[4]. It is also worth noting that a drop in a patient's albumin lowers the anion gap. A deranged phosphate level can besides bear upon the anion gap, simply to a lesser extent[4,5].
Box three: Nomenclature[two,6]
Treatment
If possible, the underlying crusade of the acid-base derangement should be treated because without doing this the trouble can recur. In some instances, it may not exist possible to treat the underlying crusade and drug treatment may exist required to correct the acid-base imbalance.
Practice examples:
Consider which blood gas disorderscould exist affecting the following patients(for reference ranges encounter Box ii, p87).
PATIENT one A 68-twelvemonth-onetime woman is admitted with abdominal pain, which is later found to be due to a pelvic abscess causing sepsis. Her arterial claret gases are as follows:
pH: 7.31
PaO2: ix.87kPa
PaCO2: 5.61kPa
HCO3–: 20.8mmol/50
Base excess: –5.two
Lactate: 1.54mmol/L
Answer This patient's pH suggests that she is acidotic. Her PaCO2 is normal and her bicarbonate is low, suggesting a metabolic acidosis. This is supported by the increased base of operations excess. Metabolic acidosis is unremarkably seen in septic patients equally a upshot of tissue hypoxia causing a build-up of lactate.
PATIENT ii A 33-year-quondam woman is admitted with H1N1 influenza and
multiple pulmonary emboli. Her arterial blood gases are equally follows:
pH: 7.55
PaO2: 14.41kPa
PaCO2: five.85kPa
HCO3–: 38.2mmol/50
Base excess: fourteen.3
Lactate: 1.87mmol/L
Reply This patient is highly alkalotic (a pH of 7.55 reflects a much greater change than if information technology had been, for example, 0.1 below normal because of the logarithmic nature of the pH scale). Her PaCO2 is normal but her bicarbonate is very high, which suggests a metabolic rather than respiratory process. The high base of operations excess also supports this. This patient was also hypokalaemic, which was driving the metabolic alkalosis (this occurs by several mechanisms including renal retentiveness of potassium ions at the expense of hydrogen ions).
References
-
1
Baylis C, Till C. Interpretation of arterial blood gases. Surgery 2009;27:470–4.
-
ii
Hennessey I, Japp A. Arterial claret gases fabricated easy. Philadelphia: : Elsevier Limited 2007.
-
3
Atherton J. Acid-base residual: maintenance of plasma pH. Amazement and Intensive Care Medicine 2009;10:557–61.
-
iv
Wargo K, Centor R. ABCs of ABGs: a guide to interpreting acrid-base disorders. Hospital Pharmacy 2008;43:808–15.
-
5
Kellum J. Disorders of acid-base remainder. Critical Intendance Medicine 2007;35:2630–6.
Source: https://pharmaceutical-journal.com/article/ld/how-to-interpret-arterial-blood-gas-results
0 Response to "The Nurse Reviews the Clients Arterial Blood Gas and Find the Ph Is 731 Pco2 Is 50 and Hco3 Is 23"
Post a Comment